missing translation for 'onlineSavingsMsg'
Learn More
Learn More
VPRBP Mouse anti-Human, Clone: 1A7A8, Proteintech
Mouse Monoclonal Antibody
1645.54 SEK - 4652.90 SEK
Specifications
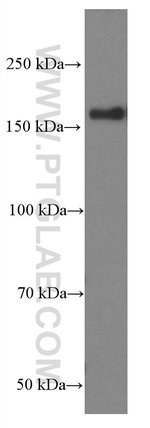
| Antigen | VPRBP |
|---|---|
| Clone | 1A7A8 |
| Concentration | 2 mg/mL |
| Applications | Western Blot, Immunohistochemistry (Paraffin) |
| Classification | Monoclonal |
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product Code | Brand | Quantity | Price | Quantity & Availability | |||||
|
16898225
|
Proteintech
66392-1-IG-20UL |
20 μL |
1645.54 SEK
20µL |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
|
16888225
|
Proteintech
66392-1-IG-150UL |
150 μL |
4652.90 SEK
150µL |
Please sign in to purchase this item. Need a web account? Register with us today! | |||||
Description
Acts both as a substrate recognition component of E3 ubiquitin-protein ligase complexes and as an atypical serine/threonine-protein kinase, playing key roles in various processes such as cell cycle, telomerase regulation and histone modification. Probable substrate-specific adapter of a DCX (DDB1-CUL4-X-box) E3 ubiquitin-protein ligase complex, named CUL4A-RBX1-DDB1-DCAF1/VPRBP complex, which mediates ubiquitination and proteasome-dependent degradation of proteins such as NF2. Involved in the turnover of methylated proteins: recognizes and binds methylated proteins via its chromo domain, leading to ubiquitination of target proteins by the RBX1-DDB1-DCAF1/VPRBP complex. The CUL4A-RBX1-DDB1-DCAF1/VPRBP complex is also involved in B-cell development: VPRBP is recruited by RAG1 to ubiquitinate proteins, leading to limit error-prone repair during V(D)J recombination. Also part of the EDVP complex, an E3 ligase complex that mediates ubiquitination of proteins such as TERT, leading to TERT degradation and telomerase inhibition. Also acts as an atypical serine/threonine-protein kinase that specifically mediates phosphorylation of ′Thr-120′ of histone H2A (H2AT120ph) in a nucleosomal context, thereby repressing transcription. H2AT120ph is present in the regulatory region of many tumor suppresor genes, down-regulates their transcription and is present at high level in a number of tumors. Involved in JNK-mediated apoptosis during cell competition process via its interaction with LLGL1 and LLGL2. In case of infection by HIV-1 virus, it is recruited by HIV-1 Vpr in order to hijack the CUL4A-RBX1-DDB1-DCAF1/VPRBP function leading to arrest the cell cycle in G2 phase, and also to protect the viral protein from proteasomal degradation by another E3 ubiquitin ligase. The HIV-1 Vpr protein hijacks the CUL4A-RBX1-DDB1-DCAF1/VPRBP complex to promote ubiquitination and degradation of proteins such as TERT and ZIP/ZGPAT. In case of infection by HIV-2 virus, it is recruited by HIV-2 Vpx in order to hijack the CUL4A-RBX1-DDB1-DCAF1/VPRBP function leading to enhanced efficiency of macrophage infection and promotion of the replication of cognate primate lentiviruses in cells of monocyte/macrophage lineage.Specifications
| VPRBP | |
| 2 mg/mL | |
| Monoclonal | |
| Liquid | |
| RUO | |
| PBS with 50% glycerol and 0.02% sodium azide; pH 7.3 | |
| DCAF1, HIV 1 Vpr binding protein, KIAA0800, Protein VPRBP, RIP, Vpr (HIV 1) binding protein, Vpr interacting protein, VPRBP | |
| DCAF1 | |
| IgG2a | |
| Protein A | |
| Antibody |
| 1A7A8 | |
| Western Blot, Immunohistochemistry (Paraffin) | |
| Unconjugated | |
| Mouse | |
| Human | |
| Q9Y4B6 | |
| 9730 | |
| VPRBP Fusion Protein Ag2184 | |
| Primary | |
| -20°C | |
| DCAF1 |
Spot an opportunity for improvement?Share a Content Correction
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
VPRBP Mouse anti-Human, Clone: 1A7A8, Proteintech