Learn More
Gibco™ PeproGMP® Human VEGF-165 Recombinant Protein, PeproTech®
Description
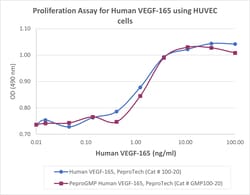
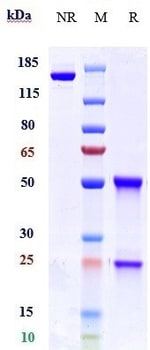
Recombinant Human VEGF 165 is a 38.2 kDa, disulfide-linked homodimeric protein consisting of two 165 amino acid polypeptide chains. Additional product information and specifications are provided on the Certificate of Analysis. All PeproGMP® products are manufactured using animal-derived component free materials. Product Description/Intended Use: PeproTech GMP recombinant proteins are manufactured for use as ancillary materials by applying applicable principles of GMP and quality control requirements from USP (United States Pharmacopeia) Chapter 1043 Ancillary Materials for Cell, Gene, and Tissue-Engineered Products. Product use statement: For research use or further manufacturing. Not for diagnostic use or direct administration into humans or animals.

Specifications
Specifications
| Accession Number | P15692 |
| For Use With (Application) | Functional Assay |
| Format | Lyophilized |
| Formulation | protein with no preservative |
| Gene ID (Entrez) | 7422 |
| Molecular Weight (g/mol) | 38.2 kDa |
| Name | PeproGMP™ Human VEGF-165 |
| Quantity | 1 mg |
| Regulatory Status | RUO |
| Endotoxin Concentration | <0.1 EU/ μg |
| Show More |
Certificates
Certificates
A lot number is required to show results for certificates. To find your lot number on previous orders use our order status area.
| Lot Number | Certificate Type | Date | Product Code |
|---|---|---|---|
| 0322G010D1422 | Certificate of Analysis | 16/07/2025 | GMP100-20-1MG |
Your input is important to us. Please complete this form to provide feedback related to the content on this product.